Scarcell Therapeutics, an innovator in cell therapies to treat chronic degenerative inflammatory diseases, recently announced the world’s first patients treated with its Allogenic Gingival Fibroblast (aGF) Therapy using gum cells from a human donor as part of its Phase Ib clinical trial studying the therapy in knee osteoarthritis (OA).
The first-in-human cases took place at Oxford University Hospitals, UK, and were led by Dr. Alex Shearman and Dr. Antony Palmer, orthopaedic surgeons and principal and co-investigator of the study, respectively, and Dr. Rajat Chowdhury, a musculoskeletal radiologist who performed the intraarticular cell injection of the knee.
OA is a complex, degenerative and incurable disease, involving multiple joint tissues and pain mechanisms. Scarcell’s patented aGF Therapy is uniquely designed to address all four of the major effects of OA – cartilage degeneration, joint inflammation, mobility and pain – in a single injection intended to be durable for up to one year. There is currently no commercial pharmaceutical or developmental cell therapy targeting all four effects.
“We are excited to lead research into this novel cell therapy for early OA, in an effort to address the unmet needs of many patients whose symptoms can be debilitating,” said Dr. Shearman. “The commencement of this trial is the result of a successful collaboration between orthopaedic surgeons, radiologists, specialist pharmacy and clinical research teams.”
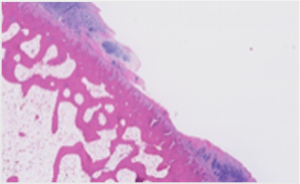
Pre-aGF Therapy histology

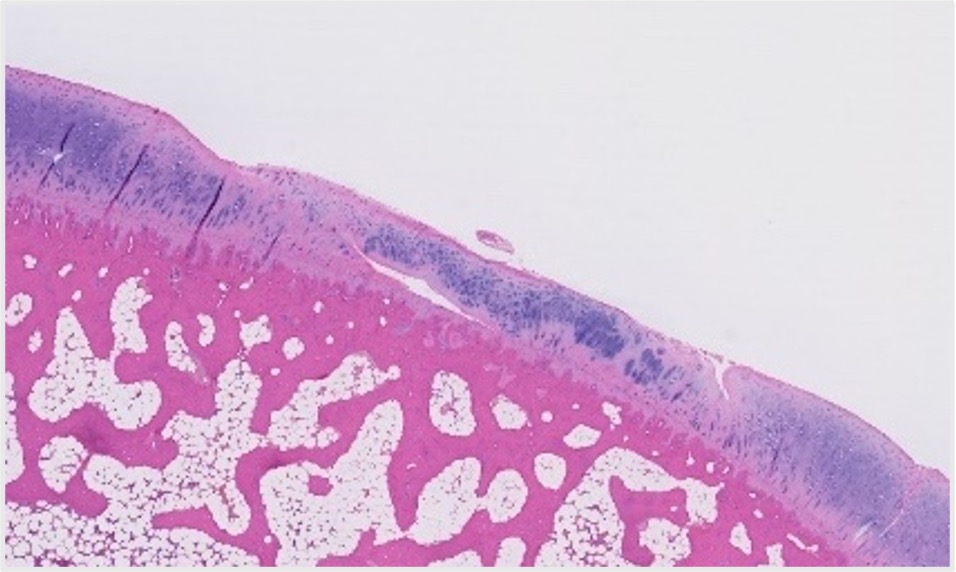
Post-aGF Therapy histology
The Phase Ib clinical trial is studying a single intra-articular injection of aGF Therapy in 15 patients treated at Oxford University Hospital. The study will examine safety, as well as knee pain, inflammation and analgesic use.
Scarcell’s aGF Therapy is designed to exhibit four mechanisms of action (MOA):
- Prevent immunologic reaction and inhibit inflammation
- Protect local cells (chondrocytes) from apoptosis (cell death) and improve extracellular matrix secretion to restore cartilage production
- Inhibit cartilage degradation
- Block nociceptive signal via TIMP1 secretion to stop pain
“Gingival cells are an exciting approach to study as donor cells do not trigger rejection by a patient’s immune system, unlike induced pluripotent stem cells (IPS) or embryonic stem cells (ES). As aGF therapy is immune tolerant, it results in cell survival and provides time for the MOAs to affect repair over several months without use of immunosuppressant drugs,” said Prof. Antoine Lafont, Professor of Medicine, Paris-Cité University, France, and founder of Scarcell Therapeutics.
“These first-in-human cases mark an exciting milestone in our mission to transform the way osteoarthritis is managed worldwide,” said Scarcell CEO Sarah Sorrel. “We are proud to lead the way with a comprehensive therapy that has the potential to provide durable relief and structural repair for millions of patients suffering from debilitating knee arthritis.”
Extensive pre-clinical study of more than 800 companion animals with severe osteoarthritis – including dogs and racehorses – receiving aGF therapy has been conducted to-date providing data on the safety and long-term effectiveness (up to two years) of a single injection.
The aGF therapy product is manufactured in Germany by Minaris (Munich, Germany) in accordance with Good Manufacturing Practice (GMP).
Source: Scarcell Therapeutics
Image: Post-aGF Therapy histology. Submitted by Scarcell Therapeutics

