Surgeon, Niel Kang of Cambridge University Hospitals NHS Trust and ODEP (Orthopaedic Data Evaluation Panel) with medical students Geethana Yogarajah and Khalid Guma’a ask the question – What has changed in the way we evaluate shoulder & elbow arthroplasty data?
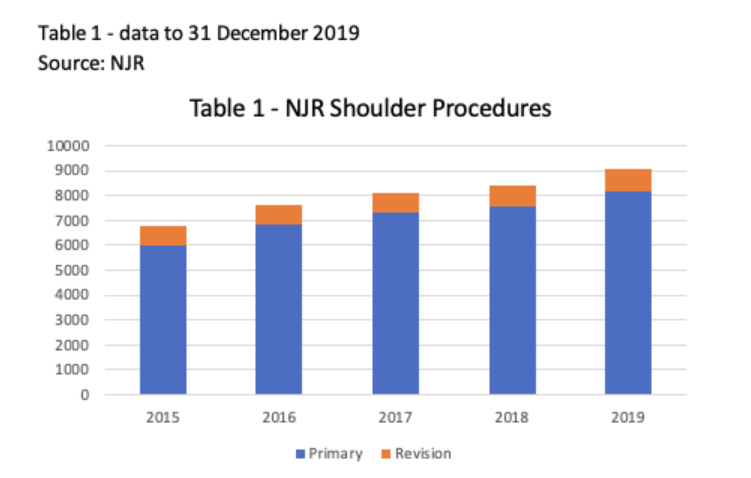
Since the formation of the ODEP Shoulder arthroplasty panel in 2017 there has been a sustained growth of shoulder arthroplasty procedures. The UK National Joint Registry (NJR) introduced shoulder arthroplasty procedures in 2012 and demonstrated significant increases (> 25-30 per cent growth annually) in implantation between 2012 and 2015, followed by steady growth in the 5 years up to the pandemic (Table 1).
Why should I care about implants?
As a patient, you can look up whether your surgeon’s operative record and the details of your procedure, – but what about the performance of the implant? Is your implant any good? This is where ODEP ratings come in. These ratings are also used by surgeons and hospital procurement to inform their decision to use specific implants.
What does the ODEP rating of my implant mean?
An ODEP rating is independent of the materials/manufacturing of the implant, but rather relies on evidence about how long the implant lasts and whether or not a reoperation has occurred.
Image 1 shows what a typical ODEP rating looks like.
A lower ODEP rating does not correlate with how good an implant is, but could instead mean that it has been in use for fewer years (early evidence is not a reliable predictor of long-term success) or not in high enough volume. A product’s ODEP rating is removed if it does not follow the expected progression through the rating benchmarks.
Image 1 showing what an ODEP rating looks like (https://www.odep.org.uk/about/rating-system/)
How does ODEP give these ratings?
- The first stage is Pre-Entry (currently 72 shoulder implants at this stage). This refers to the beginning of benchmarking (it is not a benchmark rating), when use of the implant is new, with the product not being under Beyond Compliance (a post market surveillance service) but registered with the NJR. Pre-Entry A*, on the other hand, is a benchmark rating, when the manufacturer is going through the Beyond Compliance process. This progresses to full benchmarking after the 3rd year of use, and data needs to be submitted regularly (twice a year), to progress to higher benchmarks. Once the implant implants enter the system, the manufacturer is required to submit data at the 3, 5, 7, 10, 13 and 15 year stages.The methodology has been updated recently and the key changes are:
● Type of implant determines acceptable revision rates, generally 2% above NJR and other registry revision rates for that type at a particular benchmark
● “B” rating has been modified specifically for benchmarking niche products
● Increased cohort size at each benchmark
● More standard use of Kaplan-Meier curves in the estimation of revision rate for patients where full data isn’t available for reasons such as early study termination
What progress has been made since 2017?
In addition to the redesign of the ratings methodology so that it better reflects the real-world data, lots of progress has occurred in the last 6 years.
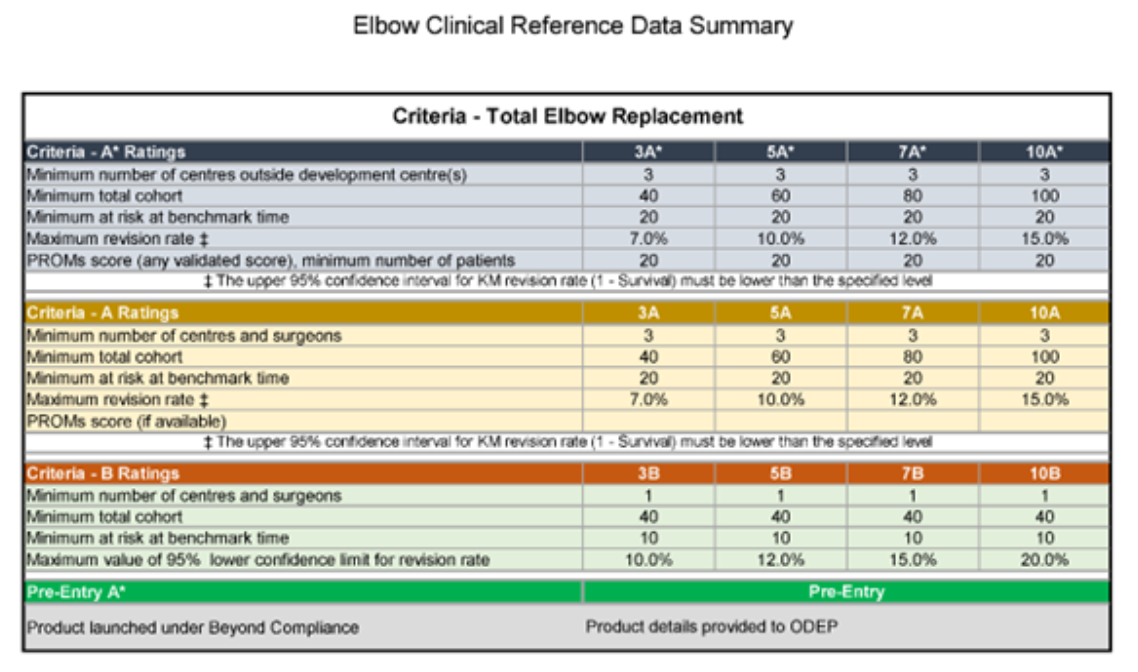
Following pilots, the first ODEP benchmarks for Trauma & resurfacing shoulder implants as well as elbow & radial head replacements are now being rated. The ODEP Elbow Criteria was released in 2021 (image 2). Since most ODEP Shoulders members are experienced with TERs (total elbow replacements), they also scrutinise elbow submissions. The challenges here are that few TERs are used per annum compared to other joints, and there are a limited number of brands.
Image 2: ODEP Elbow Criteria released in March 2021
How are shoulder/elbow ratings different to hip/knee ratings?
Revision of shoulder and elbow replacements, unlike hip and knee replacements, are uncommon and so unreliable for measuring performance/longevity and implants can be left in situ even if they fail. Total shoulder replacements, for example, are difficult to revise as only a small part of glenoid bone remains. PROMs (patient-related outcome measures) are used in the award of A* ratings of elbow and shoulder implants as revision rates alone may not paint the full picture.
Hip and knee replacements are generally revised if there are complications – if they cannot walk on the implant, this will hugely impact their life. A* ratings for hip and knee replacements rely on revision data only.
How are new implants introduced to patients?
Beyond Compliance, a sister arm of ODEP, is used to introduce some implants into UK hospitals. It works by releasing implants to a limited, predefined number of hospitals at the outset, reviewing the patient evidence and then increasing the roll out if this is safe. This enables the industry to submit new implants for recognition in a limited, regulated environment to ensure the implants are safe for wider distribution, which can then be evaluated for ‘finesse’ by ODEP.
How reliable is the data?
There is always the worry that submitted data may be inaccurate and or incomplete, and this is kept in mind by ODEP panellists. Manufacturers must answer questions about the clinical data submitted and its relevance and give reasons for omitted data. Any inconsistencies are noted with submissions for the next benchmark. So, although steps are taken to reduce this risk, ODEP will always work with the manufacturers to ensure that the submission form is complete and accurate.
How often is the methodology itself reviewed?
The methodology is reviewed every 3 – 4 years by ODEP members and an independent statistician to ensure we are on the right track.
What are the new shoulder ratings?
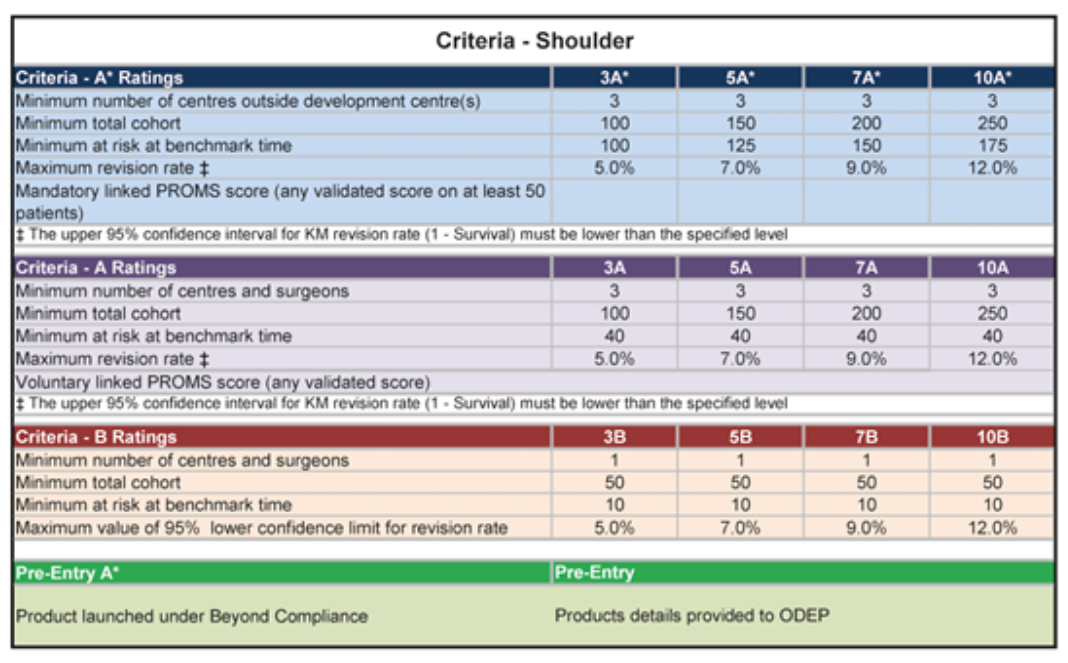
ODEP’s Shoulder policy is separate for rating stems, cups, reversed implants, resurfacing implants and trauma implants. There are many historical reasons around this, such as manufacturers producing either stems or cups but not both, making implants for specific treatments and the varying options such as cemented, uncemented and hybrid options. See image 3 for the ODEP shoulder methodology. The specifics of rating stems, glenoids, trauma stems, resurfacing implants, reversed arthroscopy constructs (humeral sockets and reversed glenoids) can be found on the website (Methodology for Shoulders – NEC ODEP). They differ in terms of what needs to be identical across the range (e.g. material composition, implant surface finish, taper/trunnion design, implant specifications) and are considered separately if can be marketed as different groups or when designed with bone defect expansions or additions e.g. “short stem” or “long stem”, reversed construct which can be adapted from anatomic to reverse etc.
Image 3: ODEP shoulder methodology published in February 2021
How do you compare implants?
The ODEP website is easy to navigate in terms of finding a product, thus enabling comparisons.
You can do an advanced search (Image 4) which enables filtering further according to product type (e.g. femoral stem, hip cup etc.), trauma implant, last review and when the next ODEP review is due. You can favourite implants of interest, generating a research list which can be shared via a link.
Image 4: Advanced search on ODEP website
What is going on elsewhere?
This is all happening with the background of reforms on medical device regulations, which includes measures to enhance transparency by increasing information captured and shared at the point of device registration. Originally the regulation was due to come into effect in the UK in 2023 but has been delayed until 2024 to minimise supply disruptions. Similar reforms are happening in Europe. They have been driven by disasters over the last two decades including the rupture-prone PIP breast implants, 3M Capital hip stem fractures, and the now-banned transvaginal mesh.
What can I do as a surgeon?
Keep engaged and familiarise yourselves with the ODEP ratings so that you can make the best decisions for your patients. If an implant does not meet the required standards, review whether the implant is suitable for use. Hip and knee surgeons can already assess their use of ODEP-rated products through their personal NJR Consultant Level Report and soon shoulder and elbow surgeons will have similar access.
Signpost manufacturers of new implants to Beyond Compliance so that they can be put on the ODEP ratings pathway, as well as any nudging manufacturers that have modified implants to register with ODEP ASSURE if they wish to maintain the rating for the original implants.
Future directions – Promoting Innovation, Augmented Implants and ODEP rated implant utilisation by surgeons
To date, it has been a stimulating and rewarding experience to help shape shoulder arthroplasty, not only at a national stage but in line with our global partners, and as a group we remain excited, working shoulder to shoulder with our industry partners to promote innovation while maintaining the safety of patients. To continue to promote innovation, ODEP has two special categories for devices without 3 years of supporting evidence: “Pre-entry” (where the manufacturer has obtained their UKCA/CE rating and registered the implant with ODEP) and “Pre-entry A*” (indicates the device has a UKCA/CE mark and is going through the Beyond Compliance process).
Manufacturers are also now also providing data and evidence of greater longevity for their implants and so the panel have extended the ratings to 13A*, 13A and 13B and will continuously review the need for further levels as demand dictates.
Since ODEP started, there has been an exponential increase in augmented glenoid implants (implants that have unique added parts to make up for abnormal anatomy) being introduced to the market. This is a trend that looks to be continuing and thus augmented implant ratings will become more prevalent.
We are also aiming to make more available the data on how many shoulder/elbow procedures a surgeon has done, how high volume, and what % is ODEP rated; this already exists in the hip and knee field as the ODEP ratings began in those fields prior to shoulder and elbow. We will continue to put in the elbow grease to ensure we provide you and your patients the best possible information, which is only possible due to the joint effort of surgeons, manufacturers, Beyond Compliance and ODEP.
Image: Canva